
MPC Pharmaceuticals is engaged in Contract Manufacturing of Finished Formulations in the form of Tablets, Capsules, Liquid Orals, and Sustained release preparations. The Company is equipped to manufacture “a wide range of pharmaceutical products in Solid & Liquid Dosage forms”.
MPC Pharmaceuticals aims to provide its customers with Novel Drug Delivery Systems and services including formulation development, Analytical method development and stability studies. The Tests are carried at par with highest standards of quality, reliability and compliance. MPC’s Success is the result of focusing on clients requirements at affordable cost & consistent quality. Customer Satisfaction / Service is on the priority list and it understands that the Association should result in its own growth and also the success of the clients. MPC Pharmaceuticals implements an unmatched global quality management system that is based on the desire to sustain a culture of operational excellence. Our company’s passion for quality goes way beyond business and statutory requirements.
MPC Pharmaceuticals has made strategic investments in common platforms of sustained release and combination product technologies, and in certain other key platforms to enhance drug delivery system capabilities. These technologies enhance the drug efficacy and safety by adopting a targeted therapy approach. The convenience of patients is an important factor while considering therapy solutions. The strategic aim is to formulate differentiated products that overcome the key challenges of conventional drug delivery systems and add value to existing products.
MPC Pharmaceuticals is committed to better healthcare for all, through quality products, achieved through manufacturing excellence, compliance to requirements and continual improvement in quality management system, for maximum customer satisfaction. Giving utmost importance to quality, MPC Pharmaceuticals has developed a sophisticated system and seamless procedure throughout the organization. This minimizes risk and enables us to produce superior quality products

QUALITY CONTROL
Quality control is an essential operation of the pharmaceutical industry. We have well experience qualified & competent dedicated technical persons in Various departments. Our QC department has all necessary instruments for analysis of API, finished products, packaging and related materials used. Quality Control refers to the features and characteristics of a product that bears on its ability to satisfy the needs of the consumer. Drugs must be marketed as safe and therapeutically active formulations whose performance is consistent and predictable.
Quality Assurance
Quality assurance department, a fully autonomous functionality is in place to keep track of each activity, parameters & processes to achieve the quality goal in totality. Quality of each medicinal product is assured by applying TQM techniques by the QA-Team. Quality assurance is a wide concept that covers all aspects that collectively or individually impact the quality of the product. That is, the sum of organized arrangements that are made with the aim of ensuring pharmaceutical products are of the required quality as per the intended use. Quality assurance is a good practice in the manufacture of pharmaceutical products, as it is the process of vouching for integrity of products to meet the standard for the proposed use. It is an obligation that ensures manufacturers meet the needs of end-user needs in terms of safety, quality, efficacy, strength, reliability and durability.
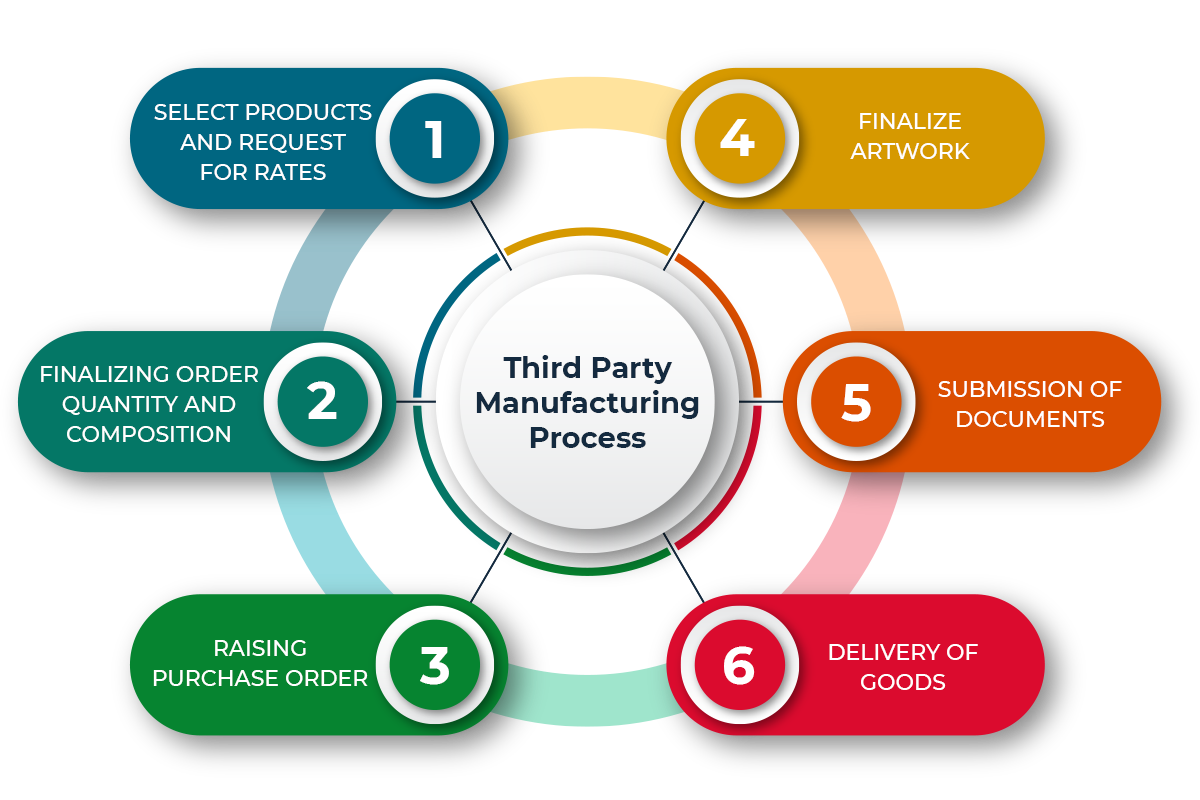
PROCESS OF THIRD PARTY MANUFACTURING







 Home
Home Range
Range Call Us
Call Us Whatsapp
Whatsapp Contact Us
Contact Us